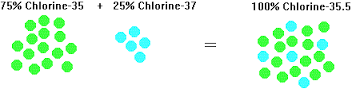isotopes and radioactivity
Key ideas and concepts
- Isotopes of an element have the same number of protons and electrons, but different numbers of neutrons.
- Different isotopes of an element have different mass numbers, but react chemically in exactly the same way.
- Mass Spectrometry is the technique used to determine the relative proportion of each isotope for an element.
- Both stable (i.e. not decaying) and unstable (radioactive) isotopes have important applications in our everyday life.
- The relative abundance of a stable isotope is the proportion of that isotope making up an element.
- The half-life of a radioactive isotope is the time taken for half of the original amount of atoms to decay.
1. What is an isotope?
You might have heard the term 'radioactive isotopes' mentioned on the news or by people worried about the environment. Sometimes we use radioactive isotopes to help treat cancer. But what is an isotope?
Each chemical element has its own unique atomic number. That means that all the atoms of any particular element must have the same number of protons (and therefore electrons). So any atom of hydrogen has one proton, otherwise it wouldn't be hydrogen! If the atom had two protons (i.e. atomic number=2), it would be helium. With 26 protons, it would be iron.
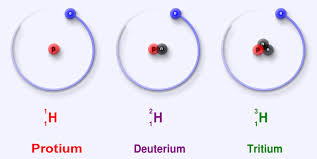
But there is no reason why the number of neutrons can't vary. In fact, there are three types of hydrogen atom, each containing a different number of neutrons. We call these isotopes of hydrogen. So isotopes are atoms of the same element that have different masses.
Each chemical element has its own unique atomic number. That means that all the atoms of any particular element must have the same number of protons (and therefore electrons). So any atom of hydrogen has one proton, otherwise it wouldn't be hydrogen! If the atom had two protons (i.e. atomic number=2), it would be helium. With 26 protons, it would be iron.
But there is no reason why the number of neutrons can't vary. In fact, there are three types of hydrogen atom, each containing a different number of neutrons. We call these isotopes of hydrogen. So isotopes are atoms of the same element that have different masses.
2. relative abundance
|
In the left handside diagram, the represented atoms only differ by the number of neutrons in the nucleus, making them isotopes of the same element. In the particular case of hydrogen, each isotope has been given a name. Each of them naturally occur in different proportion, called relative abundance. Because hydrogen is made up of a mix of three isotopes, the first isotope being by far the most abundant in the universe, its relative atomic mass in the Periodic Table is 1.007 u.
|
Here is another example: If you look up the mass of elements in a table of data, you will find that chlorine has a relative atomic mass of 35.5 u (atomic mass unit). At first sight this seems a bit strange. How can you have an atomic mass that is a fraction? After all, you can't get half a proton or half a neutron in an atom! But the answer lies in the existence of isotopes.
|
Chlorine exists as two isotopes: chlorine-35 and chlorine-37. When we calculate the relative atomic mass of an element we have to take into account the proportions of each isotope present. In a naturally occurring sample of chlorine, we find that 75 per cent is chlorine-35 atoms and the other 25 per cent is chlorine-37 atoms. Therefore, the relative atomic mass of chlorine is close to 35.5 u.
|
The isotopic composition of an element can be accurately determined by Mass Spectrometry (often abbreviated as MS). The link below provides a good introduction to the principle and applications of MS.
3. how do scientists represent isotopes?
|
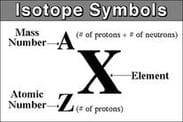
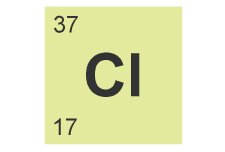
Any isotope of an element can be defined by using the A value (mass number), the Z value (atomic number) and the chemical symbol of the element X. Using the values of A and Z it is possible to calculate the number of subatomic particles within any specific isotope of an element: The number of neutrons is equal to A-Z and the number of electrons is equal Z for any neutral atom.
|
4. Use of Isotopes in real life
|
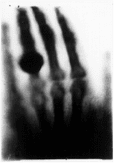
Both stable and unstable isotopes have a range of applications in real life, one of the oldest and still widely used being for the production of X-rays. This type of radiation was unexpectedly discovered by German physicist Wilhelm Roentgen while experimenting with a cathode radiation on November 8, 1895. Roentgen named X-rays (X standing for “unknown”). He took the very first picture using X-rays of his wife Anna Bertha’s hand.
|
Applications of stable isotopes
The knowledge of stable isotopes and measurement of ratios of stable isotopes is useful to a number of disciplines such as:
Click here to learn more about practical applications of stable isotopes
The knowledge of stable isotopes and measurement of ratios of stable isotopes is useful to a number of disciplines such as:
- Environmental sciences (for example, to determine the relative importance of plants and microbes to greenhouse gas emissions from soil)
- Forensic science (for example, to obtain information about the geographical, geological, chemical and/or biological provenance of materials)
- Food and drink industry (for example, to guarantee the authenticity of produce and food ingredients)
Click here to learn more about practical applications of stable isotopes
Applications of unstable isotopes
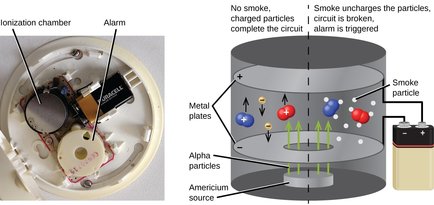
Unstable isotopes are atoms that decay spontaneously into lighter atoms by emitting a radiation, in reason of the number of neutrons their nucleus contain that makes them unstable. Another word for unstable is radioactive. For this reason, unstable isotopes are sometimes referred to as radioisotopes. The type of radiation emitted by radioisotopes is discussed in the part EXtra bits for EXperts, at the end of this section.
Several medical imagery techniques as diagnosis and treatment techniques (known as nuclear medicine) are based on the use of radioisotopes and the type of radiation they emit by decaying. Nuclear power plants and atomic bombs obtain their primary source of energy from the decay of a particular radioisotope of the element uranium.
The measurement of ratios of unstable isotopes to stable isotopes is used for dating techniques collectively known as radiometric dating or radioactive dating. These techniques are used to date materials such as rocks, in which trace radioactive impurities were selectively incorporated when they formed, or organic materials at the time the organism which they originate from died. A famous radioactive dating technique is carbon-14 dating. To learn how it works, watch the clip below.
Unstable isotopes are atoms that decay spontaneously into lighter atoms by emitting a radiation, in reason of the number of neutrons their nucleus contain that makes them unstable. Another word for unstable is radioactive. For this reason, unstable isotopes are sometimes referred to as radioisotopes. The type of radiation emitted by radioisotopes is discussed in the part EXtra bits for EXperts, at the end of this section.
Several medical imagery techniques as diagnosis and treatment techniques (known as nuclear medicine) are based on the use of radioisotopes and the type of radiation they emit by decaying. Nuclear power plants and atomic bombs obtain their primary source of energy from the decay of a particular radioisotope of the element uranium.
The measurement of ratios of unstable isotopes to stable isotopes is used for dating techniques collectively known as radiometric dating or radioactive dating. These techniques are used to date materials such as rocks, in which trace radioactive impurities were selectively incorporated when they formed, or organic materials at the time the organism which they originate from died. A famous radioactive dating technique is carbon-14 dating. To learn how it works, watch the clip below.
Click here to learn more about industrial and scientific applications of radioisotopes
5. types of radioactive decay
When the nucleus of an atom possesses either too many or too few neutrons compared to the number of protons it becomes unstable and tries to reach a stable form. This is achieved by emitting a radiation (energy and matter). Atoms with unstable nuclei are called radioactive isotopes.
There are mainly three types of decay, represented by a greek letter. To each decay type correspond a particle emitted that has the same name. These particles were identified on the basis of their electrical charge
Alpha decay
Beta decay
The process of alpha or beta decay lead to the isotope of another element that may either be stable or unstable and hence lead to another decay cycle.
Gamma decay
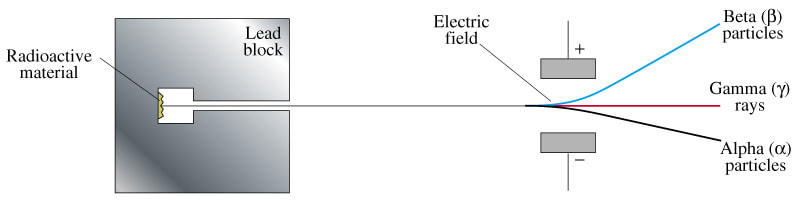
The image below shows how each of these particles react when passing in an electrical field. Beta particles are deflected in the direction of a positive electrode, therefore it has to be negatively charged. It was later found to be an electron. Alpha particles are attracted towards the negative electrode, indicating that they are positively charged. Gamma rays pass through the electrical field undeflected, as would be expected for a particle having no electrical charge.
There are mainly three types of decay, represented by a greek letter. To each decay type correspond a particle emitted that has the same name. These particles were identified on the basis of their electrical charge
Alpha decay
- The nucleus emits an alpha particle, that is a particle containing two protons and two neutrons.
- This means the atomic mass number decreases by 4 and the atomic number decreases by 2. A new element is formed that is two places lower in the Periodic Table than the original element.
- Alpha particles are relatively heavy particles and therefore have a short range in air. They can easily be blocked by air, a sheet of paper or even skin. However Alpha particles can cause some damage at short distance, for example if swallowed, where they would damage internal organs and tissues.
- Some isotopes of the element radon undergo alpha decay. Radon is a noble gas found in many rocks. If a lot of radon is inhaled, it can get in your lungs and damage lung tissue. Some homes are even equipped with radon detectors to warn you if the levels are getting too high. An isotope of uranium, known as uranium-238 also undergoes alpha decay.
Beta decay
- It occurs when a neutron changes into a proton and an electron. The proton stays in the nucleus and the electron leaves the atom with high energy, and we call it a beta particle. Therefore, a beta particle is really just a high-energy electron being emitted from a nucleus.
- When a beta particle is emitted, the nucleus has one more proton and one less neutron. This means the atomic mass number remains unchanged while the atomic number increases by 1. A new element is formed that is two places lower in the Periodic Table than the original element
- Beta decay occurs when the nucleus contains too many neutrons. Unlike alpha paticles, beta particles are very light and move at high speed. They can travel through paper but are generally stopped by the first layers of skin. When ingested, they can produce tissue damage and cancer. However, they can also be used to kill cancerous cells.
- An example of electron emission by beta decay is the decay of carbon-14 into nitrogen-14. Note that another type of beta decay occurs when a nucleus contains too many protons to be stable.
The process of alpha or beta decay lead to the isotope of another element that may either be stable or unstable and hence lead to another decay cycle.
Gamma decay
- Energy in the form of gamma radiation or rays are emitted from the nucleus. Gamma radiation are electromagnetic waves (a type of light) with very high frequencies and energy.
- Gamma decay has no effect on either the atomic number, therefore it does not lead to a new element.
- After alpha or beta decay, nuclei often rearrange themselves. This process causes the loss of energy in the form of gamma rays. Therefore, gamma decay normally occurs after an alpha or beta decay.
- Gamma rays have a high penetrating power and can cause damage at the cellular level. Shielding from gamma rays requires large amounts of mass, in contrast to alpha particles, which can be blocked by paper or skin, and beta particles, which can be shielded by foil.
The image below shows how each of these particles react when passing in an electrical field. Beta particles are deflected in the direction of a positive electrode, therefore it has to be negatively charged. It was later found to be an electron. Alpha particles are attracted towards the negative electrode, indicating that they are positively charged. Gamma rays pass through the electrical field undeflected, as would be expected for a particle having no electrical charge.
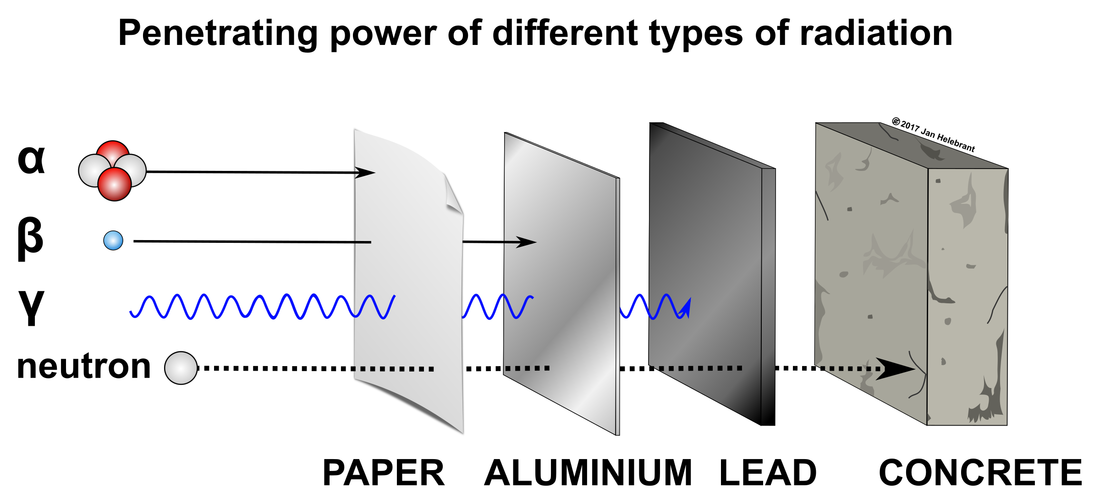
Comparison of penetrating powers
Alpha particles may be completely stopped by a sheet of paper while beta particles require at least aluminium shielding. Gamma rays can only be reduced by much more substantial mass, such as a very thick layer of lead or concrete.
Neutron emission
Another type of decay is spontaneous fission where very heavy atoms spontaneously break down into lighter atoms, releasing a lot of energy and emitting neutrons. Because of their neutral electrical charge and high energy, neutrons are very penetrating, but their penetrating power depends on their energy. The isotope 235 of uranium, used in nuclear power plants and nuclear bombs, undergoes this type of decay. A type of imaging technique uses neutrons to see the internal structure of an object. For example, it can see through layers of paint in a painting to help work out who created it.
Another type of decay is spontaneous fission where very heavy atoms spontaneously break down into lighter atoms, releasing a lot of energy and emitting neutrons. Because of their neutral electrical charge and high energy, neutrons are very penetrating, but their penetrating power depends on their energy. The isotope 235 of uranium, used in nuclear power plants and nuclear bombs, undergoes this type of decay. A type of imaging technique uses neutrons to see the internal structure of an object. For example, it can see through layers of paint in a painting to help work out who created it.
Did you know?
Gamma rays and X-rays are identical in nature: they are both a form of high energy light. The difference is that X-rays are artificially produced (from the forced movement of electrons between electron shells) while gamma rays are naturally occurring during the decay of a radioactive isotope.
Gamma rays and X-rays are identical in nature: they are both a form of high energy light. The difference is that X-rays are artificially produced (from the forced movement of electrons between electron shells) while gamma rays are naturally occurring during the decay of a radioactive isotope.
6. half-life of unstable isotopes
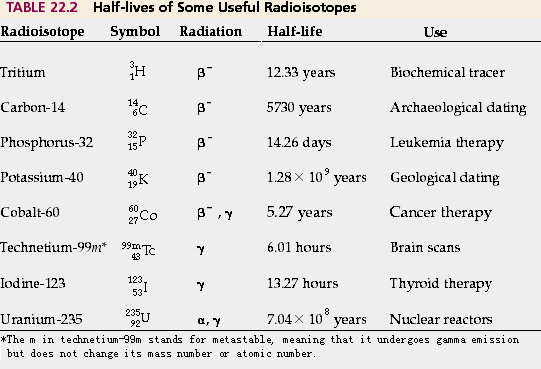
The time it takes for an amount of radioisotope to decay by half its original amount into lighter elements is known as its half-life. Although it is not possible to predict when a particular atom of a radioactive isotope will decay, the average time it takes for 50 % of a large number of atoms to decay can be measured. The half-life of a radioisotope can vary from milliseconds to longer than the age of the universe. Some examples of half-life of widely used isotopes are given in the table below.
As a practial example, the radioactive decay of carbon-14 has a half-life of 5,730 years. A quantity of carbon-14 will decay to half of its original amount (on average) after 5,730 years, regardless of how big or small the original quantity was.
The predictability of the time it takes for a certain percentage of the original amount to decay is what makes the technique of radiodating so powerful and ubiquitous, from dating billions of years old geological layers in the earth crust or a few hundred years old objects with high accuracy.
The predictability of the time it takes for a certain percentage of the original amount to decay is what makes the technique of radiodating so powerful and ubiquitous, from dating billions of years old geological layers in the earth crust or a few hundred years old objects with high accuracy.
PRACTICAL ACTIVITY #1: BUILD YOUR OWN ISOTOPES
Learn how to make isotopes by using the simulation below. Follow instruction in the worksheet provided by your teacher.
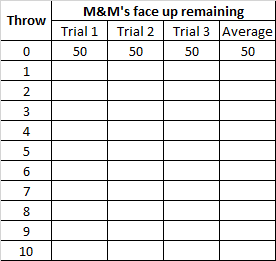
Work in pairs. Reproduce the table in your notebook before you start.
- Each group is starting with 50 M&M’s candies in a zip lock bag, which is recorded as Throw 0 in the data table. All of the M&M’s candies are radioactive.
- Gently shake the bag and spill out the candies onto a flat surface.
- Sort the candies with the “m” showing - these are still radioactive - from the others.
- Count the “m” candies as you return them to the bag and record this number of candies in the table.
- Move the candies that are blank on the top to the side - these have now decayed to a stable state (do not eat them!).
- Repeat steps 2 through 5 until all the candies have decayed. Add more rows to the table if necessary.
- Perform 3 trials and calculate the average number of M&M's for each throw.
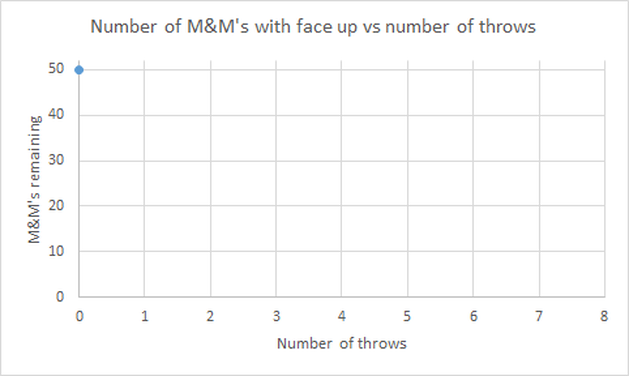
- Plot the average number of M&M's remaining after each throw against the number of throws, using the graph below as model. Compare the graph you obtained with other groups and wait for your teacher's instructions.